Silver got its name from Anglo-Saxon word seolfe meaning silver.
2. This is how silver looks like as a pure element.

3. Silver have 47 protons and 47 electrons.
Below is a table of the isotope, atomic mass, and natural abundance.
The average atomic mass is 107.8682 amu.
109Ag 108.904756 (4) 48.161 (7) |
4. There are 47 electrons in a neutral atom of silver.
It has five shells of electrons.
5. Atomic radius of silver in meters is: 144 picometers = 1.44 × 10-10 meters.
6. The properties of silver in its pure elemental form: soft, white metal with a shiny surface. It is malleable ,
conducts heat and reflect light.
conducts heat and reflect light.
7. Below is a table of the abundance in silver in grams (g).
| Location | ppb by weight | ppb by atoms |
|---|---|---|
| Universe | 0.6 | 0.007 |
| Sun | 1 | 0.01 |
| Meteorite (carbonaceous) | 140 | 20 |
| Crustal rocks | 80 | 20 |
| Sea water | 0.1 | 0.0057 |
| Stream | 0.3 | 0.003 |
| Human | no data | no data |
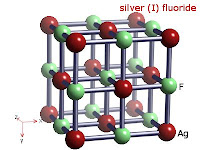
8. Compounds that silver form is: Silver fluoride: AgF, Silver chloride: AgCl, and Silver bromide: AgBr.
9. "Slag dumps in Asia Minor and on islands in the Aegean Sea indicate that man learned to separate silver
from lead as early as 3000 B.C."
10. Silver is used in jewelery, tableware and utensils, photography, dental alloys, solder and brazing alloys.
11. You contain 0g of SILVER!!!
12. CLICK HERE =>>> www.webelements.com


No comments:
Post a Comment